
By following the height of the amorphous peak and the height of the 220 cubic diffraction maximum during a gradual 1K/min warmup, we find that the ice starts to change before crystallization has its onset. The change starts at the glass transition temperature (T = 120-140 K). At that point, the ice film breaks into a number of droplets, minimizing the contact area with the hydrophobic amorpous carbon film.
When warmed higher, the amorphous ice film crystallizes to cubic crystalline ice. The crystallization occurs in two steps: a rapid crystal growth driven phase and a more slow nucleation-rate limited phase. The crystal domains remain small and occupy only a fraction of each droplet.
When the diffraction peaks finally cease to become more intense, only a fraction of the ice has transformed. Most of the ice stays in an amorphous form, which we call the restrained amorphous form. The radial distribution function of the amorphous form just before onset of crystallization shows some relaxation of strained bonds after passing the glass transition temperature.
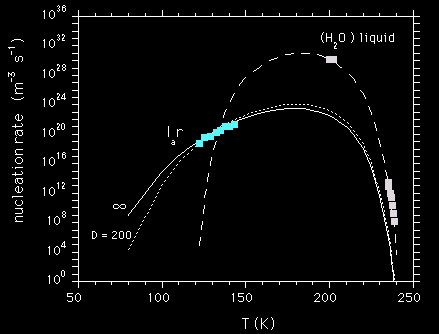
The speed of crystallization is driven by the viscosity of the amorphous form. We find that the crystallization rate changes gradually with temperature. Hence, the restrained amorphous ice remains highly viscous at high temperature. That behaviour is consistent with a strong liquid, not with a fragile liquid such as liquid water.
The strong/fragile nature of water ice above the glass transition temperature depends on impurity content. Impure ices can stay in an amorphous form for longer periods of time at higher temperature than pure water ice. This implies that amorphous ice may even exist on the poles of Mercury for millions of years, if temperatures do not go above T = 90 K in the shade of craters.
Last modified: July 7, 1997
P. Jenniskens
D.F. Blake